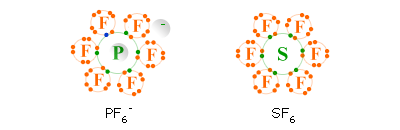
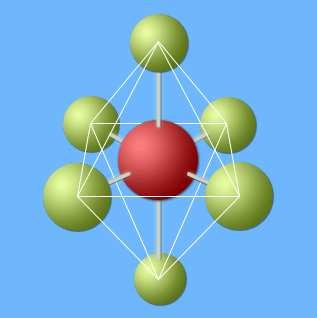
A compound with four electron pairs at its central atom has a tetrahedral shape.
If one of the four electron pairs is unshared or non-bonding, then the compound becomes pyramidal in shape.
Although triangular pyramid is also a tetrahedron, there is a distinction between compounds with tetrahedral and pyramidal shapes: the location of their central atoms.
A compound with triangular pyramidal or simply pyramidal shape has its central atom located at the apex of the pyramid whereas a compound with tetrahedral shape has its central atom located at the center of tetrahedron.
Here are the lists of compounds included in this post:
| compounds with tetrahedral shapes |
| CH4 |
| F3SN |
| CCl4 |
| ClO4- |
| NH4+ |
| BF4- |
| SO4= |
| compounds with pyramidal shapes |
| NH3 |
| SO3= |
| ClO3- |
| NF3 |
| XeO3 |
| F2SeO |
Along with the illustration of a compound's shape, its Lewis structure is also included together with a brief discussion regarding the effect of lone electron pairs or double/triple bonds on the bond angles and/or the use of hybrid orbitals by certain atoms for covalent bonding.
The first three compounds discussed in details below serve to represent the rest of the compounds.
Methane, CH4
- shape of molecule: tetrahedral
- H-C-H bond angle: 109.5°
The Lewis structure and one of the four equal H-C-H bond angles of CH
4.
A tetrahedron superimposed on a CH
4 molecule. The four hydrogen atoms are oriented towards the corners of the tetrahedron.
Ammonia, NH3
The ammonia molecule has a non-bonding electron pair at the top of the tetrahedron as depicted below.
The Lewis structure of NH
3 shows it has a lone pair of electrons which occupies more space than any of the bonding electron pairs resulting to H-N-H bond angles being less than the ideal tetrahedral angle of 109.5°.
Since molecular shapes involve atoms only, the shape of ammonia will be minus the lone pair of electrons. It is a triangular pyramid with the nitrogen atom at the apex.
- shape of molecule: triangular pyramidal
- H-N-H bond angle: 106.6°
A triangular pyramid superimposed on the ammonia molecule.
Trifluorothionitrile, F3S≡N
- shape of molecule: tetrahedral
- F-S-F bond angle: less than 109.5°
- N-S-F bond angle: greater than 109.5°
Referring to the Lewis structure above, sulfur atom exceeds the octet configuration or the eight valence electrons required for bonding by utilizing empty d orbitals and creating hybrid orbitals (for a discussion of constructing Lewis structure of compounds, see
Drawing Lewis Electron Dot Structure or Formula).
F
3SN has a tetrahedral shape which means it has four electron pairs directed towards the four corners of a tetrahedron but its Lewis structure shows 6 bonding electron pairs (see my
previous post for a discussion of compounds' different coordination geometries and the corresponding number of electron pairs of their central atoms).
In determining the shapes of compounds,
only electron pairs involved in sigma bonds are considered.
As for the bond angles, greater repulsion exerted by electron pairs from double and triple bonds cause the bond angles containing them to be greater than those bond angles containing single bonds only.
Compounds With Tetrahedral Shape
The Geometry of CCl
4
The Lewis structures of CCl
4 and ClO
4-.
The chlorine atom in ClO
4- or perchlorate ion acquires one electron to complete its eight valence electrons and utilizes empty d orbitals to bond with four oxygen atoms by sharing all of its eight valence electrons.
Referring to the illustration above, atoms in partial grey and red shades are atoms with formal charges. To learn more about the calculation of formal charges, read
Drawing Lewis Electron Dot Structure or Formula.
The Geometry of ClO
4-
The Geometry of NH
4+
The Lewis structures of NH
4+ and BF
4-.
The NH
4+ or ammonium ion is formed by NH
3 acting as a Bronsted base and accepting a proton.
On the other hand, BF
4- or boron tetrafluoride ion is formed by BF
3 acting as a Lewis acid and bonding with a fluoride ion acting as a Lewis base.
For a discussion of Bronsted acid-base reactions, see
Solving Weak Acid/Base Dissociation Problems.
The Geometry of BF
4-
The Geometry of SO
4=
The Lewis structures of SO
4=
Given the geometry of SO
4= or sulfate ion as shown above, there are two possible Lewis structures for it. The second Lewis structure is considered to be the correct one because it is more stable and consistent with the experimental data.
The sulfur atom in the second Lewis structure exceeds the octet configuration or the 8 valence electrons required for bonding by acquiring two extra electrons and utilizing empty d orbitals.
Compounds With Pyramidal Shape
The Geometry of SO
3=
The Lewis structures of SO
3= and ClO
3-
The sulfur and chlorine atoms in the above Lewis structures acquire extra electrons to fill up its valence electron shells and are assumed to use hybrid orbitals involving empty d orbitals to form covalent bonds with oxygen atoms.
The Geometry of ClO
3-
The Geometry of NF
3
The Lewis structures of NF
3 and XeO
3
The Geometry of XeO
3
The Geometry of F
2SeO
The Lewis structure of F
2SeO